Algunos asistentes y miembros redGDPS en el congreso
Un año más asistimos al congreso de la EASD ( Sociedad Europea de Diabetes) celebrado en Barcelona, en su 49 edición.
Próximamente se publicará un monográfico en la revista Diabetes Práctica con las novedades más interesantes del evento.
En twitter se han recogido algunos comentarios con el #eads2013 @redGDPS.
Se puede acceder a una gran parte de las comunicaciones en la web del congreso de forma libre, así como a power point y streaming de las conferencias.
Aqui presentamos una comunicacion en forma de póster presentada durante el congreso de nuestros compañeros de la redGDPS, en abierto a través de la web del congreso.
Enlace : http://www.easdvirtualmeeting.org/users/2507
The cost of type 2 diabetes: a population-based study in Catalonia
Authors: I. Vinagre1, M. Mata-Cases2,3, J. Franch-Nadal4,3, M. Casajuana3, R. Morros3,5, E. Hermosilla3, F. Fina3, C. Castell6, B. Bolivar3,5, D. Mauricio7;.
1Service de Diabetologie, Centre Hospitalier Sud Francilien, Corbeil Essonne, 2Université Pierre & Marie Curie, Paris, 3INSERM, Villejuif, 4Novo Nordisk, Paris,5University of Franche-Comté, Besançon, 6Toulouse University Hospital, 7Saint Louis Hospital, Paris, France.
1Service de Diabetologie, Centre Hospitalier Sud Francilien, Corbeil Essonne, 2Université Pierre & Marie Curie, Paris, 3INSERM, Villejuif, 4Novo Nordisk, Paris,5University of Franche-Comté, Besançon, 6Toulouse University Hospital, 7Saint Louis Hospital, Paris, France.
Background and aims: Baseline and 1-year data from a post-marketing study in France were compared to assess the change in glycaemic control and body weight after 1 year’s treatment with the human GLP-1 analogue liraglutide in patients who previously received a DPP-4 inhibitor (DPP-4i).
Materials and methods: EVIDENCE is a multicentre, observational, post-marketing outpatient study requested by the French National Health Authority to evaluate the efficacy and safety of liraglutide. Its primary objective is to determine the percentage of patients still taking liraglutide and at HbA1c target (<7%) after 2 years.
Statistical analyses: for quantitative variables a normality Kolmogorov-Smirnov test was used; comparisons of two independent groups were performed using the Mann-Whitney Wilcoxon test for quantitative variables and chi-square test for qualitative variables; for paired quantitative variables the Wilcoxon signed rank test was used; for paired qualitative variables the McNemar test was used.
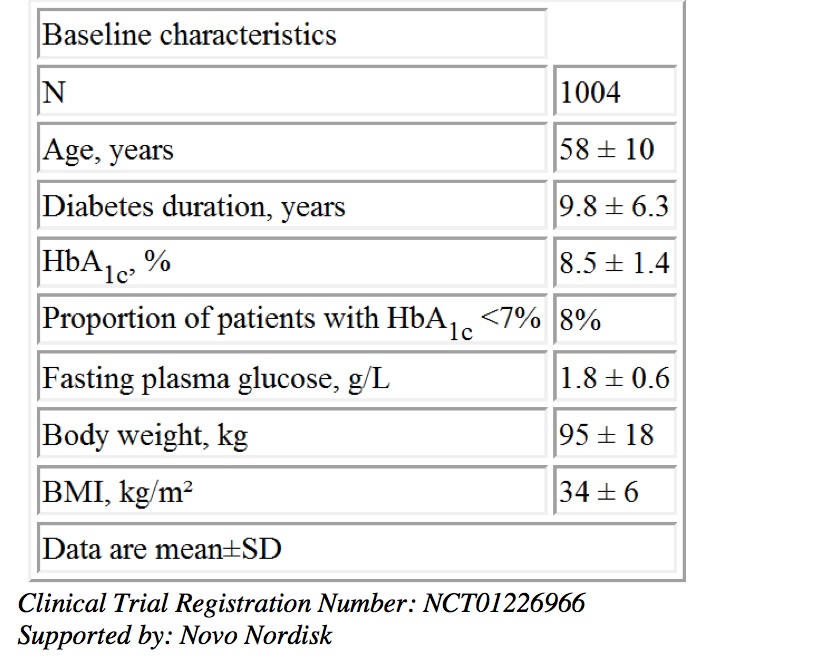
Results: Data were collected from 3137 subjects of whom 1255 (40%) were receiving a DPP-4i prior to liraglutide initiation. A total of 1004 (32%) subjects switched from a DPP-4i to liraglutide at study start, while 251 (8%) added liraglutide to a DPP-4i. The table shows baseline characteristics of subjects who switched. Subjects who switched and continued liraglutide treatment for 1 year (n=734) achieved significant reductions from baseline in mean HbA1c (-0.84%, p<0.0001), fasting plasma glucose (-0.27 g/L, p<0.0001) and body weight (-3.53 kg, p<0.0001). A greater percentage of subjects had HbA1c <7% at 1 year (31.6%) vs. baseline (8.0%; p<0.0001). Withdrawals (30% in overall study cohort) were mostly due to gastrointestinal disorders experienced early in the study.
Conclusion: In this observational study, significant improvements in glycaemic control and body weight from baseline were seen after 1 year of liraglutide treatment in a subgroup of patients who switched from a DPP-4i at study start. A limitation of this study is the lack of control arm, making it difficult to evaluate whether observed improvements are attributable to liraglutide alone. It was previously shown by Pratley et al. in 2012 that switching from the DPP-4i sitagliptin to liraglutide in a randomised controlled trial setting provided significant HbA1c (-0.5%; p<0.0001) and body weight reductions (-2.5 kg; p<0.0001). The cohort enrolled in EVIDENCE is more representative of patients routinely treated in the clinic; therefore results reported here may provide a better indication of the outcomes of liraglutide treatment in patients who switch from a DPP-4i in daily clinical practice.
Materials and methods: EVIDENCE is a multicentre, observational, post-marketing outpatient study requested by the French National Health Authority to evaluate the efficacy and safety of liraglutide. Its primary objective is to determine the percentage of patients still taking liraglutide and at HbA1c target (<7%) after 2 years.
Statistical analyses: for quantitative variables a normality Kolmogorov-Smirnov test was used; comparisons of two independent groups were performed using the Mann-Whitney Wilcoxon test for quantitative variables and chi-square test for qualitative variables; for paired quantitative variables the Wilcoxon signed rank test was used; for paired qualitative variables the McNemar test was used.
Results: Data were collected from 3137 subjects of whom 1255 (40%) were receiving a DPP-4i prior to liraglutide initiation. A total of 1004 (32%) subjects switched from a DPP-4i to liraglutide at study start, while 251 (8%) added liraglutide to a DPP-4i. The table shows baseline characteristics of subjects who switched. Subjects who switched and continued liraglutide treatment for 1 year (n=734) achieved significant reductions from baseline in mean HbA1c (-0.84%, p<0.0001), fasting plasma glucose (-0.27 g/L, p<0.0001) and body weight (-3.53 kg, p<0.0001). A greater percentage of subjects had HbA1c <7% at 1 year (31.6%) vs. baseline (8.0%; p<0.0001). Withdrawals (30% in overall study cohort) were mostly due to gastrointestinal disorders experienced early in the study.
Conclusion: In this observational study, significant improvements in glycaemic control and body weight from baseline were seen after 1 year of liraglutide treatment in a subgroup of patients who switched from a DPP-4i at study start. A limitation of this study is the lack of control arm, making it difficult to evaluate whether observed improvements are attributable to liraglutide alone. It was previously shown by Pratley et al. in 2012 that switching from the DPP-4i sitagliptin to liraglutide in a randomised controlled trial setting provided significant HbA1c (-0.5%; p<0.0001) and body weight reductions (-2.5 kg; p<0.0001). The cohort enrolled in EVIDENCE is more representative of patients routinely treated in the clinic; therefore results reported here may provide a better indication of the outcomes of liraglutide treatment in patients who switch from a DPP-4i in daily clinical practice.